Malonic Esters
Diethyl malonate is also called malonic ester.
Preparation:-
Diethyl malonate is prepared very conveniently by boiling sodium or potassium Cyano-acetate with alcohol and concentrated hydrochloric acid.
Physical properties :-
Diethyl malonate is a colorless, pleasant smelling liquid, BP 199.2° C . It is sparingly soluble in water, freely soluble in alcohol and ether.
Chemical properties :-
Acidity of CH2 group, Formation of salts :-
--------------Like ethyl acetoacetate, diethyl malonate contains a methylene group joined to two carbonyl groups. the H atoms of the CH2 group are acidic. This is attributed to two factors:
1. The electron attracting power of the electronegative oxygen of the carbonyl group (Inductive effect).
2. The resonance stabilization of the resultant cation (diethyl malonate anion) Thus,
The diethyl malonate anion is highly resonance stabilized so that it's negative charge is delocalized into the two carbonyl groups.
Salt Formation :-
The CH2 group is being sufficiently acidic, diethyl malonate reacts with a strong base like sodium ethoxide (C2H5ONa) to form the sodium salt.
Alkylation :-
Diethyl malonate anion is nucleophilic. it reacts with halides to give diethyl alkyl malonate
Hydrolysis and decarboxylation :-
Diethyl malonate undergoes hydrolysis with dilute HCl to give malonic acid, Similarly diethyl alkyl malonate gives alkyl malonic acids such acids that have two -COOH groups separated by a carbon on heating (at 150° C) split out a molecule of CO2 to give the monocarboxylic acid




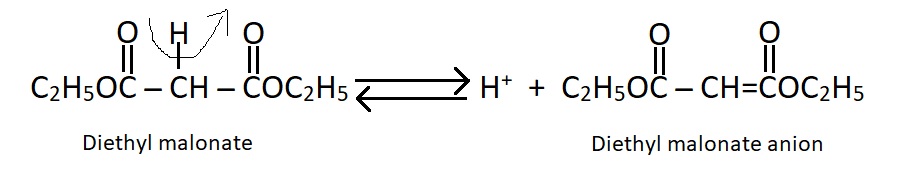















0 Comments
Thanks for your feedback, ll get back to you soon