HYDROGEN BONDING
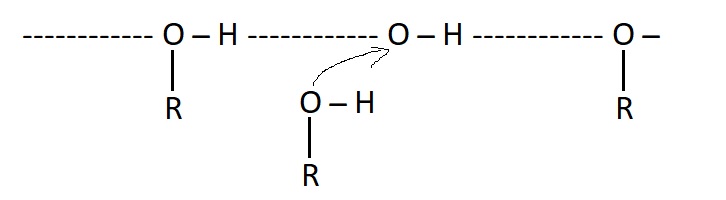
Hydrogen bonding is said to be an attractive force which occurs in any compound whose molecule containing O ̶ H, N ̶ H (Water alcohols amides) OH bond is highly polar bond, oxygen is more electronegative than hydrogen and it acquires small negative charge, hydrogen atom has positive charge.
R ̶ O ̶ H+
Adjacent molecules of the compound containing an O ̶ H bond will be attracted to each other by opposite charges, this forces of attraction is known as "Hydrogen bonding"
Hydrogen bonding have a significant effect on the physical properties of organic compounds.
EFFECT ON BOILING POINTS:-
It is easy to understandable that substances having nearly the same molecular weights have the same boiling point.
CH3 ̶ CH2
̶
CH3 CH3
̶
O ̶ CH3 CH3 ̶ CH2
̶ OH
Explaining on the basis of hydrogen bonding, Ethanol forms hydrogen bonds. extra energy in the form of heat is required to break the hydrogen bonds holding the molecules together before it can be volatilized. Propyl and di-methyl ether do not form hydrogen bonds and therefore they have low boiling points.
EFFECT ON WATER SOLUBILITY:-
A hydrogen bond substance is usually soluble in another hydrogen bond substance. alcohols are soluble in water but the alkanes are insoluble in water because non-polar alkane molecule cannot break into the hydrogen bond sequence in a water molecule that's why alkanes are insoluble in in water.
Puvvukonvict..............















0 Comments
Thanks for your feedback, ll get back to you soon