Free radicals of carbon :-
Carbonium ions and carbanions, these have no charge they are formed by homolytic fission.
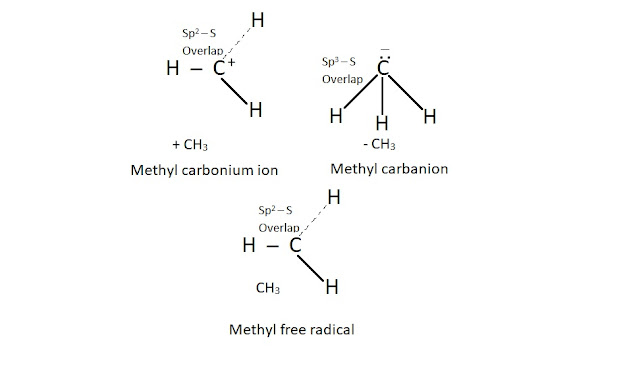
The carbon atom in a carbon free radical uses Sp2 hybrid orbitals to form three σ bonds. A half filled P orbital extends above and below the plane of σ bonds. Thus carbon free radicals are electrically neutral and have one unpaired electron associated with them.
They are extremely reactive because of the tendency of this electron to become paired at the earliest opportunity, free radicals combine with other free radicals or with other molecules to produce larger free radicals. carbon free radicals are named after the parent alkyl group and adding the word free radical. For example,
Like carbonium ions, a tertiary free radical is more stable than a secondary and the secondary is more stable than the primary. Free radicals are also stabilized by resonance.
Puvvukonvict................................















0 Comments
Thanks for your feedback, ll get back to you soon