Mutarotation is a phenomenon observed in carbohydrates, particularly in sugars, where there is a change in the optical rotation of a solution due to the interconversion between different anomeric forms of the sugar (α and β forms). This occurs in cyclic sugars like glucose, where the sugar exists in an equilibrium between its open-chain form and two different cyclic hemiacetal forms (α and β).
Principle of Mutarotation:
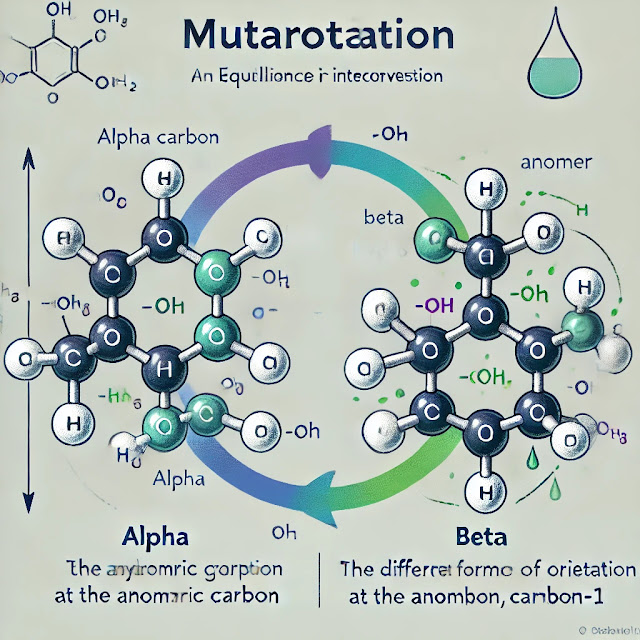
Cyclic Forms of Sugars: Many monosaccharides, like glucose, exist in a cyclic form in solution. For D-glucose, the most stable forms are the cyclic hemiacetals known as α-D-glucopyranose and β-D-glucopyranose.
Anomeric Carbon: When a sugar cyclizes, the carbonyl carbon (C1 in aldoses like glucose) becomes a new chiral center called the anomeric carbon. This can lead to two possible configurations at this carbon:
- α-anomer: The hydroxyl group on the anomeric carbon is on the opposite side (trans) of the ring relative to the CH₂OH group (C6 in glucose).
- β-anomer: The hydroxyl group on the anomeric carbon is on the same side (cis) of the ring relative to the CH₂OH group.
Interconversion in Solution: In an aqueous solution, these two anomers (α and β) can interconvert through the open-chain (linear) form of the sugar. This process is dynamic, and both forms exist in equilibrium.
- The sugar first opens up into its linear (open-chain) form.
- The open-chain form then cyclizes again, but it can form either the α or β anomer.
Optical Rotation Change: Each of these anomeric forms has a different specific optical rotation. For example:
- α-D-glucose has a specific rotation of about +112°.
- β-D-glucose has a specific rotation of about +19°.
When either pure α-D-glucose or β-D-glucose is dissolved in water, the optical rotation changes over time until it reaches an equilibrium value (~+52.7° for D-glucose) due to the establishment of a dynamic equilibrium between the α and β forms. This change in optical rotation is called mutarotation.
Structures Involved in Mutarotation:
Let's consider D-glucose as an example.
Open-chain form (linear form of D-glucose): The open-chain form contains an aldehyde group (at C1) which is reactive and can cyclize to form a ring.
Cyclic α-D-glucopyranose (α-anomer): In this structure, the C1 hydroxyl group is on the opposite side of the ring relative to the CH₂OH group on C5.
Cyclic β-D-glucopyranose (β-anomer): In this structure, the C1 hydroxyl group is on the same side of the ring as the CH₂OH group.
Mechanism of Mutarotation:
- Start with α-D-glucose: When pure α-D-glucose is dissolved in water, it starts to open up into the linear (open-chain) form.
- Equilibrium: The open-chain form can then close to form either the α- or β-anomer, leading to a mixture of the two forms in solution.
- Final equilibrium: Over time, a mixture is formed, and the optical rotation settles at a value corresponding to the ratio of the α- and β-anomers.
Summary:
- Mutarotation is the change in optical rotation due to the interconversion between α and β forms of a sugar in solution.
- This occurs because sugars exist in equilibrium between their open-chain form and two cyclic anomeric forms.
- D-glucose provides a classic example, where the specific rotation changes as equilibrium between α-D-glucopyranose and β-D-glucopyranose is established.















0 Comments
Thanks for your feedback, ll get back to you soon