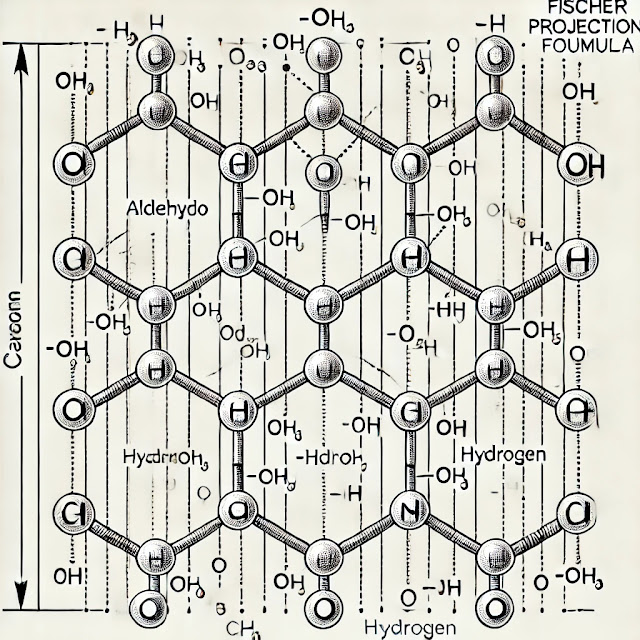
The Fischer projection is a two-dimensional representation used to depict the three-dimensional structure of molecules, especially carbohydrates. It was developed by Hermann Emil Fischer, a German chemist, and is particularly useful for showing the stereochemistry of sugars.
Key Features of Fischer Projections:
Orientation:
- The carbon chain is drawn vertically, with the most oxidized carbon (usually the carbonyl group in aldehydes or ketones) at the top.
- Each intersection in the vertical line represents a carbon atom.
Stereochemistry:
- Horizontal lines represent bonds that project out of the plane of the paper (towards the viewer).
- Vertical lines represent bonds that project behind the plane of the paper (away from the viewer).
- This convention helps to easily determine the configuration of chiral centers.
D and L Notation:
- The D or L designation of a carbohydrate refers to the configuration of the chiral carbon furthest from the carbonyl group (usually the penultimate carbon in the chain).
- If the hydroxyl group on this chiral carbon is on the right side in the Fischer projection, the molecule is labeled as D. If it is on the left side, it is labeled as L.
Aldoses and Ketoses:
- Aldoses have an aldehyde group (-CHO) at the top (C1), while ketoses have a ketone group (usually at C2).
- Common examples of aldoses include glucose and mannose, while fructose is a common ketose.
Application:
- Fischer projections are particularly useful for comparing different isomers of carbohydrates. For instance, D-glucose and D-galactose differ only in the configuration of the hydroxyl group at C4.
- It also simplifies the understanding of reactions that involve carbohydrates, such as glycosidic bond formation or epimerization.
Examples:
D-Glucose
CHO
|
H-C-OH
|
OH-C-H
|
H-C-OH
|
H-C-OH
|
CH2OH
D-Fructose
CH2OH
|
C=O
|
HO-C-H
|
H-C-OH
|
H-C-OH
|
CH2OH
In summary, the Fischer projection is a vital tool in carbohydrate chemistry, providing a clear way to represent the stereochemistry of sugars and other biomolecules, facilitating the understanding of their structure and reactivity.












0 Comments
Thanks for your feedback, ll get back to you soon