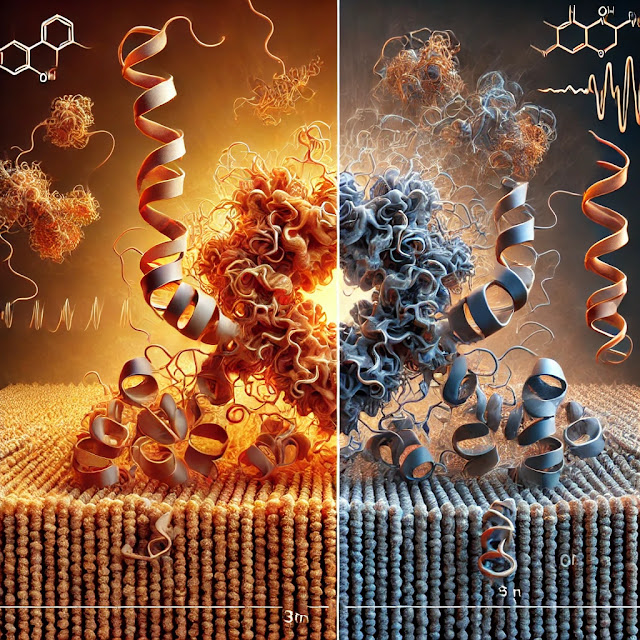
Denaturation of proteins is the process where a protein loses its natural three-dimensional structure. Proteins have a specific structure held together by various types of bonds, like hydrogen bonds, ionic bonds, disulfide bridges, and hydrophobic interactions. This structure is essential for their function. When denaturation occurs, these bonds break, leading to an unfolded or misfolded structure, which typically results in a loss of biological function.
Causes of Protein Denaturation
- Temperature: High temperatures can increase molecular movement, causing the bonds that maintain the protein structure to break.
- pH Changes: Extreme acidic or alkaline conditions can disrupt ionic bonds and change the charge on amino acids, leading to structural alterations.
- Chemical Agents: Chemicals like urea, alcohol, and guanidine hydrochloride can disrupt hydrogen bonds and hydrophobic interactions.
- Mechanical Stress: Vigorous mixing or stirring can unfold proteins by disrupting weak interactions.
Effects of Denaturation
- Loss of Function: Enzymes, for example, lose their active sites and can no longer catalyze reactions.
- Aggregation: Denatured proteins may clump together, leading to aggregation due to exposure of hydrophobic regions that are usually buried within the protein.
- Irreversibility: In many cases, denaturation is irreversible, meaning the protein cannot return to its functional state. However, mild denaturation can sometimes be reversible.
Importance of Understanding Denaturation
In fields like biochemistry and pharmacology, knowing about denaturation helps in understanding how to preserve protein-based drugs, store enzymes, and understand diseases related to protein misfolding, such as Alzheimer's and Parkinson's.












0 Comments
Thanks for your feedback, ll get back to you soon