Buffers
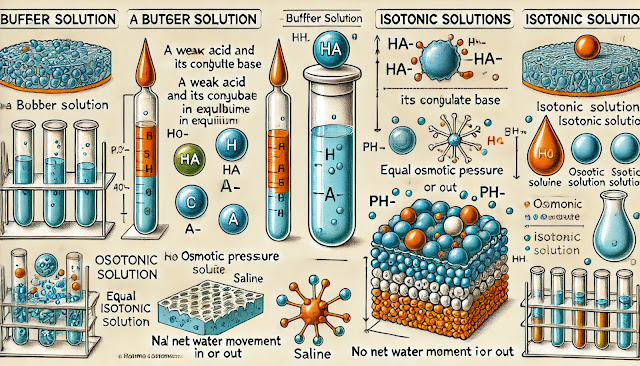
Definition: A buffer is a solution that can resist pH changes upon the addition of small amounts of either acid or base. It usually consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
Definition: A buffer is a solution that can resist pH changes upon the addition of small amounts of either acid or base. It usually consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
How Buffers Work:
- Weak Acid and Conjugate Base: For example, a common buffer solution is made of acetic acid (CH₃COOH) and sodium acetate (CH₃COONa). In water, acetic acid partially dissociates into H⁺ and CH₃COO⁻ ions. Sodium acetate fully dissociates into Na⁺ and CH₃COO⁻ ions.
- Buffer Action: When a small amount of acid (H⁺ ions) is added to the buffer solution, the conjugate base (CH₃COO⁻) reacts with the added H⁺ ions to form CH₃COOH, thus minimizing the change in pH.
- Addition of Base: When a small amount of base (OH⁻ ions) is added, the weak acid (CH₃COOH) reacts with OH⁻ to form water and CH₃COO⁻ ions, again minimizing the change in pH.
Applications:
- Biological Systems: Blood is a buffer solution, maintaining a pH of around 7.4.
- Industrial Processes: Buffers are used in fermentation processes, pharmaceuticals, and chemical manufacturing.
Isotonic Solutions
Definition: An isotonic solution has the same osmotic pressure as another solution, typically a biological fluid. For cells, an isotonic solution has the same solute concentration as the cytoplasm, preventing the net movement of water into or out of the cell.
Key Points:
- Osmotic Pressure: It is the pressure required to prevent the flow of water across a semipermeable membrane. Isotonic solutions have equal osmotic pressure, so there is no net water movement.
- Common Isotonic Solutions: Normal saline (0.9% NaCl) and lactated Ringer's solution are commonly used isotonic solutions in medical settings.
- Cellular Impact: When cells are placed in an isotonic solution, they retain their normal shape and function, as there is no net gain or loss of water.
Applications:
- Medical Uses: Isotonic solutions are used for intravenous infusions to hydrate patients without causing cells to shrink (crenate) or swell (lyse).
- Laboratory Experiments: Isotonic solutions are used to maintain cell integrity in experiments involving cell cultures.
Summary
- Buffers maintain a stable pH in a solution by neutralizing small amounts of added acid or base. They are essential in many biological and chemical processes.
- Isotonic Solutions have equal osmotic pressure to biological fluids, preventing net water movement across cell membranes, and are crucial in medical treatments to maintain cellular health.












0 Comments
Thanks for your feedback, ll get back to you soon